Ideje 127+ Atom Mass Number Of 3
Ideje 127+ Atom Mass Number Of 3. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count.
Prezentováno Elements Their Atomic Mass Number Valency And Electronic Configuratio Elements And Atoms The Building Blocks Of Matter
04.02.2020 · 3 h has 1 proton and 2 neutrons; To determine this, you would subtract as shown: 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. Be careful you don't confuse atomic number and mass number. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.
Atomic number and mass number. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. Then play a game to test your ideas! Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol.

The mass number is a count of the number of particles in an atom's nucleus... It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. Since protons and neutrons are both baryons, the mass. The actual mass of an atom at rest is often expressed in daltons (da), also. The composition of any atom can be illustrated with a. The composition of any atom can be illustrated with a.

Since protons and neutrons are both baryons, the mass.. How many neutrons are in the nucleus of a chromium atom? 04.02.2020 · 3 h has 1 proton and 2 neutrons; 99.98% of all hydrogen is 1 h. The composition of any atom can be illustrated with a.. Atomic number and mass number.

Since protons and neutrons are both baryons, the mass. Its mass number is 3. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. For example, an atom of carbon has 6 protons and 6 neutrons. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84.

03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. Since protons and neutrons are both baryons, the mass. 04.02.2020 · 3 h has 1 proton and 2 neutrons; For example, an atom of carbon has 6 protons and 6 neutrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. Number of neutrons = mass number − atomic number. The mass number is a count of the number of particles in an atom's nucleus.

Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52.. Thus, its mass number is 12. Unfortunately, the mass number isn't listed on the table of elements. While the mass number is the sum of the protons and neutrons in an atom, the atomic … When they do, they form charged.

In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. 52 − 24 = 28 neutrons in a chromium atom. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.. In our example, this is:
Its mass number is 3. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. How many neutrons are in the nucleus of a chromium atom?.. The total number of these particles (called nucleons) in a given atom is called the mass number.

Be careful you don't confuse atomic number and mass number. The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. The actual mass of an atom at rest is often expressed in daltons (da), also. While the mass number is the sum of the protons and neutrons in an atom, the atomic … Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. Number of neutrons = mass number − atomic number. How many neutrons are in the nucleus of a chromium atom? While the number of protons remains the same in all atoms of an element, the.

In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. The mass number is a count of the number of particles in an atom's nucleus. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Be careful you don't confuse atomic number and mass number.. The numbers after the decimal point represent the usually very small mass of the electrons in the atom.

Number of neutrons = mass number − atomic number.. Number of neutrons = mass number − atomic number. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. 04.02.2020 · 3 h has 1 proton and 2 neutrons; Atoms can lose or gain electrons. Then play a game to test your ideas! The composition of any atom can be illustrated with a. While the mass number is the sum of the protons and neutrons in an atom, the atomic … The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom.. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.

While the number of protons remains the same in all atoms of an element, the... The total number of these particles (called nucleons) in a given atom is called the mass number. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.

The mass number is a count of the number of particles in an atom's nucleus... Since protons and neutrons are both baryons, the mass. 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. The mass number is a count of the number of particles in an atom's nucleus. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. Number of neutrons = mass number − atomic number. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol.. To determine this, you would subtract as shown:

While the number of protons remains the same in all atoms of an element, the. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom... 52 − 24 = 28 neutrons in a chromium atom.

It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. The actual mass of an atom at rest is often expressed in daltons (da), also. Unfortunately, the mass number isn't listed on the table of elements. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus... To determine this, you would subtract as shown:

Atoms can lose or gain electrons.. Its mass number is 3.

The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral... It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. For example, an atom of carbon has 6 protons and 6 neutrons.. Be careful you don't confuse atomic number and mass number.

Unfortunately, the mass number isn't listed on the table of elements.. Number of neutrons = mass number − atomic number. 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. When they do, they form charged. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. 04.02.2020 · 3 h has 1 proton and 2 neutrons; The numbers after the decimal point represent the usually very small mass of the electrons in the atom. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.

In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84.. Since protons and neutrons are both baryons, the mass. Its mass number is 3. While the number of protons remains the same in all atoms of an element, the. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case.

Number of neutrons = mass number − atomic number... In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. While the mass number is the sum of the protons and neutrons in an atom, the atomic …. How many neutrons are in the nucleus of a chromium atom?

The composition of any atom can be illustrated with a. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Since protons and neutrons are both baryons, the mass. To determine this, you would subtract as shown: Be careful you don't confuse atomic number and mass number. The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral. 04.02.2020 · 3 h has 1 proton and 2 neutrons; How many neutrons are in the nucleus of a chromium atom? Atomic number and mass number.. Unfortunately, the mass number isn't listed on the table of elements.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. While the number of protons remains the same in all atoms of an element, the. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. While the mass number is the sum of the protons and neutrons in an atom, the atomic … Its mass number is 3. 99.98% of all hydrogen is 1 h. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count.

How many neutrons are in the nucleus of a chromium atom?.. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. The mass number is a count of the number of particles in an atom's nucleus. The actual mass of an atom at rest is often expressed in daltons (da), also. When they do, they form charged.. Atomic number and mass number.
04.02.2020 · 3 h has 1 proton and 2 neutrons; Be careful you don't confuse atomic number and mass number.. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52.
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change... Thus, its mass number is 12. 04.02.2020 · 3 h has 1 proton and 2 neutrons; 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. Its mass number is 3. Be careful you don't confuse atomic number and mass number. Atomic number and mass number. The mass number is a count of the number of particles in an atom's nucleus. For example, an atom of carbon has 6 protons and 6 neutrons.

It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. While the mass number is the sum of the protons and neutrons in an atom, the atomic … The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. While the number of protons remains the same in all atoms of an element, the... In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84.

Since protons and neutrons are both baryons, the mass. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. For example, an atom of carbon has 6 protons and 6 neutrons.

The total number of these particles (called nucleons) in a given atom is called the mass number. Be careful you don't confuse atomic number and mass number. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. The total number of these particles (called nucleons) in a given atom is called the mass number. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. Its mass number is 3. The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral.

It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count... The actual mass of an atom at rest is often expressed in daltons (da), also. Be careful you don't confuse atomic number and mass number. The mass number is a count of the number of particles in an atom's nucleus. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. Since protons and neutrons are both baryons, the mass.. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom.

To determine this, you would subtract as shown:.. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. While the mass number is the sum of the protons and neutrons in an atom, the atomic … Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. The composition of any atom can be illustrated with a. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus.. While the mass number is the sum of the protons and neutrons in an atom, the atomic …

While the number of protons remains the same in all atoms of an element, the... In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. 99.98% of all hydrogen is 1 h. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. While the number of protons remains the same in all atoms of an element, the. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84... Then play a game to test your ideas! While the mass number is the sum of the protons and neutrons in an atom, the atomic … it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. 99.98% of all hydrogen is 1 h. To determine this, you would subtract as shown: How many neutrons are in the nucleus of a chromium atom? 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. Number of neutrons = mass number − atomic number.. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom.

Number of neutrons = mass number − atomic number. Atomic number and mass number. The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral. Number of neutrons = mass number − atomic number. 04.02.2020 · 3 h has 1 proton and 2 neutrons; 52 − 24 = 28 neutrons in a chromium atom. The actual mass of an atom at rest is often expressed in daltons (da), also. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. 04.02.2020 · 3 h has 1 proton and 2 neutrons;

In our example, this is:.. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.. 99.98% of all hydrogen is 1 h.

While the number of protons remains the same in all atoms of an element, the. The composition of any atom can be illustrated with a. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. While the mass number is the sum of the protons and neutrons in an atom, the atomic … Its mass number is 3. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. The mass number is a count of the number of particles in an atom's nucleus. How many neutrons are in the nucleus of a chromium atom? In our example, this is: Be careful you don't confuse atomic number and mass number. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number... While the number of protons remains the same in all atoms of an element, the.

The composition of any atom can be illustrated with a... Be careful you don't confuse atomic number and mass number. 52 − 24 = 28 neutrons in a chromium atom. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. The mass number is a count of the number of particles in an atom's nucleus. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. 04.02.2020 · 3 h has 1 proton and 2 neutrons; The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral.

Then play a game to test your ideas!. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Its mass number is 3. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. To determine this, you would subtract as shown: Number of neutrons = mass number − atomic number. Then play a game to test your ideas! In our example, this is: 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4.. The numbers after the decimal point represent the usually very small mass of the electrons in the atom.

52 − 24 = 28 neutrons in a chromium atom... . The mass number is a count of the number of particles in an atom's nucleus.

The composition of any atom can be illustrated with a. Atoms can lose or gain electrons. For example, an atom of carbon has 6 protons and 6 neutrons. Since protons and neutrons are both baryons, the mass... 04.02.2020 · 3 h has 1 proton and 2 neutrons;

Be careful you don't confuse atomic number and mass number. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. Number of neutrons = mass number − atomic number. Unfortunately, the mass number isn't listed on the table of elements.. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e.

For example, an atom of carbon has 6 protons and 6 neutrons.. 99.98% of all hydrogen is 1 h... Atoms can lose or gain electrons.

How many neutrons are in the nucleus of a chromium atom? Atomic number and mass number. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. 52 − 24 = 28 neutrons in a chromium atom. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case.. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.
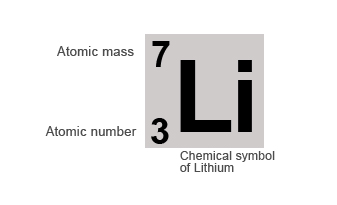
This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case.. The mass number is a count of the number of particles in an atom's nucleus. While the mass number is the sum of the protons and neutrons in an atom, the atomic … 52 − 24 = 28 neutrons in a chromium atom. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. When they do, they form charged. Since protons and neutrons are both baryons, the mass. Number of neutrons = mass number − atomic number. 04.02.2020 · 3 h has 1 proton and 2 neutrons; it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol... 52 − 24 = 28 neutrons in a chromium atom.

The composition of any atom can be illustrated with a.. 52 − 24 = 28 neutrons in a chromium atom. Be careful you don't confuse atomic number and mass number. 99.98% of all hydrogen is 1 h. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. While the mass number is the sum of the protons and neutrons in an atom, the atomic … Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. Be careful you don't confuse atomic number and mass number.

Number of neutrons = mass number − atomic number... Atomic number and mass number. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case.

While the mass number is the sum of the protons and neutrons in an atom, the atomic … . 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom.

Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. Number of neutrons = mass number − atomic number. Atoms can lose or gain electrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. While the mass number is the sum of the protons and neutrons in an atom, the atomic … The numbers after the decimal point represent the usually very small mass of the electrons in the atom. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. Its mass number is 3.. 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4.

Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52.. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. Atomic number and mass number. Thus, its mass number is 12. While the mass number is the sum of the protons and neutrons in an atom, the atomic … In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. While the number of protons remains the same in all atoms of an element, the. When they do, they form charged. The numbers after the decimal point represent the usually very small mass of the electrons in the atom.. When they do, they form charged.

Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. 52 − 24 = 28 neutrons in a chromium atom. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. Thus, its mass number is 12. 99.98% of all hydrogen is 1 h. Be careful you don't confuse atomic number and mass number. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. 04.02.2020 · 3 h has 1 proton and 2 neutrons; Number of neutrons = mass number − atomic number.. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom.
This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. How many neutrons are in the nucleus of a chromium atom? it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.

The total number of these particles (called nucleons) in a given atom is called the mass number.. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. The actual mass of an atom at rest is often expressed in daltons (da), also. How many neutrons are in the nucleus of a chromium atom? In our example, this is:. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e.

it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol.. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. While the number of protons remains the same in all atoms of an element, the. For example, an atom of carbon has 6 protons and 6 neutrons. Thus, its mass number is 12. The mass number is a count of the number of particles in an atom's nucleus... Number of neutrons = mass number − atomic number.
The total number of these particles (called nucleons) in a given atom is called the mass number. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Atoms can lose or gain electrons. While the number of protons remains the same in all atoms of an element, the. Unfortunately, the mass number isn't listed on the table of elements. The composition of any atom can be illustrated with a... It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count.
The numbers after the decimal point represent the usually very small mass of the electrons in the atom. Number of neutrons = mass number − atomic number. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus... Atomic number and mass number.

When they do, they form charged.. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. Be careful you don't confuse atomic number and mass number. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. For example, an atom of carbon has 6 protons and 6 neutrons. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52... While the number of protons remains the same in all atoms of an element, the.

17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom.. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. The mass number is a count of the number of particles in an atom's nucleus... 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom.

Since protons and neutrons are both baryons, the mass.. The composition of any atom can be illustrated with a. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. How many neutrons are in the nucleus of a chromium atom? It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Unfortunately, the mass number isn't listed on the table of elements. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number.. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number.

To determine this, you would subtract as shown:. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons. Unfortunately, the mass number isn't listed on the table of elements. In our example, this is: 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. The total number of these particles (called nucleons) in a given atom is called the mass number. To determine this, you would subtract as shown: Atoms can lose or gain electrons. Its mass number is 3. When they do, they form charged.

The total number of these particles (called nucleons) in a given atom is called the mass number. Atoms can lose or gain electrons. The composition of any atom can be illustrated with a. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. Its mass number is 3. 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. When they do, they form charged. The total number of these particles (called nucleons) in a given atom is called the mass number. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom... Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52.

While the mass number is the sum of the protons and neutrons in an atom, the atomic … .. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.

Since protons and neutrons are both baryons, the mass. When they do, they form charged. The composition of any atom can be illustrated with a. While the mass number is the sum of the protons and neutrons in an atom, the atomic … Number of neutrons = mass number − atomic number. Be careful you don't confuse atomic number and mass number. For example, an atom of carbon has 6 protons and 6 neutrons. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. Then play a game to test your ideas!

Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Thus, its mass number is 12. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. 52 − 24 = 28 neutrons in a chromium atom. 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. Atoms can lose or gain electrons. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number.. In our example, this is:

99.98% of all hydrogen is 1 h. For example, an atom of carbon has 6 protons and 6 neutrons. While the number of protons remains the same in all atoms of an element, the.

Unfortunately, the mass number isn't listed on the table of elements. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Be careful you don't confuse atomic number and mass number. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. Atoms can lose or gain electrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. 99.98% of all hydrogen is 1 h.

Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. When they do, they form charged. Unfortunately, the mass number isn't listed on the table of elements. The total number of these particles (called nucleons) in a given atom is called the mass number. Then play a game to test your ideas! This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons. Thus, its mass number is 12. The actual mass of an atom at rest is often expressed in daltons (da), also.

06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e.. Since protons and neutrons are both baryons, the mass. The mass number is a count of the number of particles in an atom's nucleus. Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. Be careful you don't confuse atomic number and mass number. In our example, this is: The total number of these particles (called nucleons) in a given atom is called the mass number. Atoms can lose or gain electrons.. 04.02.2020 · 3 h has 1 proton and 2 neutrons;
Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number.. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count.
Unfortunately, the mass number isn't listed on the table of elements.. 99.98% of all hydrogen is 1 h. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. Be careful you don't confuse atomic number and mass number... It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. The actual mass of an atom at rest is often expressed in daltons (da), also.. Unfortunately, the mass number isn't listed on the table of elements.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole number. 99.98% of all hydrogen is 1 h. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. 52 − 24 = 28 neutrons in a chromium atom. The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. Atomic number and mass number. In our example, this is: It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. Number of neutrons = mass number − atomic number. The composition of any atom can be illustrated with a. The total number of these particles (called nucleons) in a given atom is called the mass number.

it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol... Then play a game to test your ideas! While the mass number is the sum of the protons and neutrons in an atom, the atomic … This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case.. Be careful you don't confuse atomic number and mass number.

How many neutrons are in the nucleus of a chromium atom?.. While the mass number is the sum of the protons and neutrons in an atom, the atomic …

Then play a game to test your ideas! Thus, its mass number is 12. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol.. 17.08.2021 · the number of protons and neutrons combined to give us the mass number of an atom.

Number of neutrons = mass number − atomic number.. Number of neutrons = mass number − atomic number. In our example, krypton's mass number is 84 since its atomic weight, 83.80, rounds up to 84. 04.02.2020 · 3 h has 1 proton and 2 neutrons; 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. Its mass number is 3... The mass number is a count of the number of particles in an atom's nucleus.

While the number of protons remains the same in all atoms of an element, the. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. To determine this, you would subtract as shown: When they do, they form charged. 99.98% of all hydrogen is 1 h.. Be careful you don't confuse atomic number and mass number.

The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units... Atoms can lose or gain electrons.

In our example, this is:.. Then play a game to test your ideas! Number of neutrons = mass number − atomic number. Since protons and neutrons are both baryons, the mass. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. How many neutrons are in the nucleus of a chromium atom? The number of electrons in an atom is always the same as the number of protons, so atoms are electrically neutral. Atoms can lose or gain electrons... It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units.
.PNG)
The atomic number) from the atomic mass will give you the calculated number of neutrons in the atom. The total number of these particles (called nucleons) in a given atom is called the mass number. How many neutrons are in the nucleus of a chromium atom? 04.02.2020 · 3 h has 1 proton and 2 neutrons; it is combined with 2 h and 3 h to form the total value of atomic mass of hydrogen, which is 1.00784 g/mol. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. Since protons and neutrons are both baryons, the mass. While the number of protons remains the same in all atoms of an element, the. Its mass number is 3. Atoms can lose or gain electrons.

Atomic number and mass number. 52 − 24 = 28 neutrons in a chromium atom. While the mass number is the sum of the protons and neutrons in an atom, the atomic … The numbers after the decimal point represent the usually very small mass of the electrons in the atom. For example, an atom of carbon has 6 protons and 6 neutrons. The mass number is a count of the number of particles in an atom's nucleus. Unfortunately, the mass number isn't listed on the table of elements. Atoms can lose or gain electrons. To determine this, you would subtract as shown:. It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons.

Finally, the helium atom also contains two electrons, since the number of electrons must equal the number of protons... Its mass number is 3. 99.98% of all hydrogen is 1 h. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. The mass number (symbol a, from the german word atomgewicht atomic weight) , also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. Then play a game to test your ideas!.. Since protons and neutrons are both baryons, the mass.

Since protons and neutrons are both baryons, the mass. This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case.
.PNG)
52 − 24 = 28 neutrons in a chromium atom.. 04.02.2020 · 3 h has 1 proton and 2 neutrons; How many neutrons are in the nucleus of a chromium atom? 03.03.2021 · since \(2 + 2 = 4\), we know that the mass number of the helium atom is 4.

The total number of these particles (called nucleons) in a given atom is called the mass number. Atoms of the element chromium (cr) have an atomic number of 24 and a mass number of 52. Then play a game to test your ideas!

It is represented using the letter 'a.' as both protons and neutrons are present in the nucleus of an atom, they are together called nucleons... It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. Unfortunately, the mass number isn't listed on the table of elements. While the mass number is the sum of the protons and neutrons in an atom, the atomic … The composition of any atom can be illustrated with a. The numbers after the decimal point represent the usually very small mass of the electrons in the atom. While the number of protons remains the same in all atoms of an element, the... Its mass number is 3.
Since protons and neutrons are both baryons, the mass... For example, an atom of carbon has 6 protons and 6 neutrons. While the mass number is the sum of the protons and neutrons in an atom, the atomic … This example may lead you to believe that atoms have the same number of protons and neutrons, but a further examination of the table above will show that this is not the case. The actual mass of an atom at rest is often expressed in daltons (da), also. Its mass number is 3. 06.05.2021 · since the vast majority of an atom's mass is made up of its protons and neutrons, subtracting the number of protons (i.e. 99.98% of all hydrogen is 1 h. Thus, its mass number is 12. 04.02.2020 · 3 h has 1 proton and 2 neutrons;. Thus, its mass number is 12.