Nápady Carbon Atom Notation Čerstvé
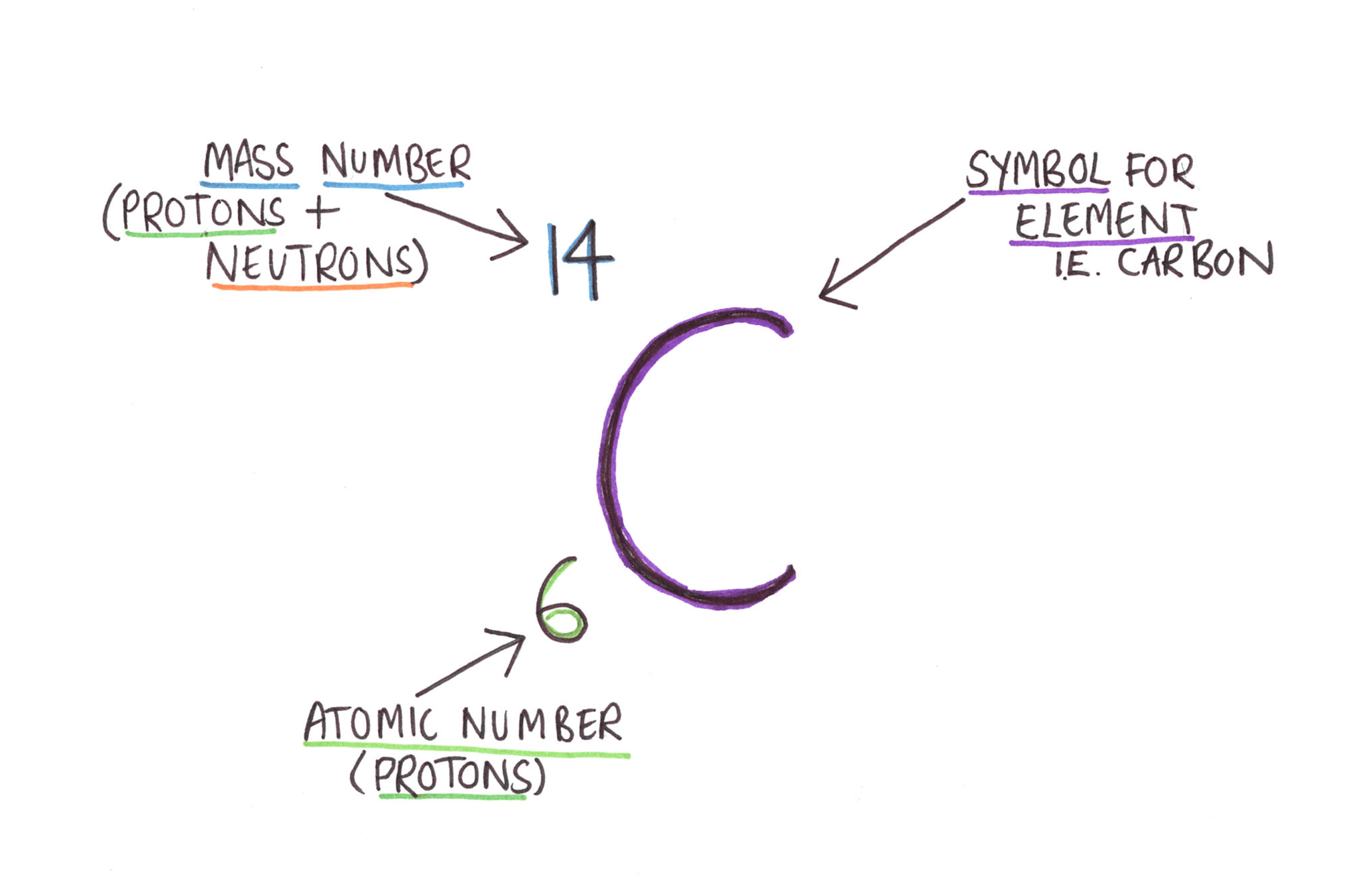
Nápady Carbon Atom Notation Čerstvé. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: How to write the electron configuration for carbon.
Nejchladnější Term Symbol Wikipedia
Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. The orbital diagram shows how the electrons are arranged within each sublevel.Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei.
Its electron configuration is 1s^22s^22p^2. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Electronegativity of carbon is 2.55. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.
Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol... Nuclear notation heavy element synthesis The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. If the atom is neutral, it will have the same number of negatively charged electrons. Additionally, what is the noble gas notation of carbon?

Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Additionally, what is the noble gas notation of carbon? Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome.. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c.

The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. How to write the electron configuration for carbon. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Additionally, what is the noble gas notation of carbon? The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons... If the atom is neutral, it will have the same number of negatively charged electrons.

Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers... Nuclear notation heavy element synthesis Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Additionally, n = a −z. Its electron configuration is 1s^22s^22p^2. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times.. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.

Its electron configuration is 1s^22s^22p^2.. Additionally, n = a −z. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Additionally, what is the noble gas notation of carbon? The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Its electron configuration is 1s^22s^22p^2. The maximum number of electrons allowed in an orbital is 2, each with. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. How to write the electron configuration for carbon.. The orbital diagram shows how the electrons are arranged within each sublevel.

The maximum number of electrons allowed in an orbital is 2, each with.. How to write the electron configuration for carbon. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Its electron configuration is 1s^22s^22p^2. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. How to write the electron configuration for carbon.

If the atom is neutral, it will have the same number of negatively charged electrons. Additionally, n = a −z. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. How to write the electron configuration for carbon. Electronegativity of carbon is 2.55. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c... Electronegativity of carbon is 2.55.

If the atom is neutral, it will have the same number of negatively charged electrons... .. If the atom is neutral, it will have the same number of negatively charged electrons.

Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons... The maximum number of electrons allowed in an orbital is 2, each with. If the atom is neutral, it will have the same number of negatively charged electrons. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j.
.PNG)
Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol... Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. If the atom is neutral, it will have the same number of negatively charged electrons. The maximum number of electrons allowed in an orbital is 2, each with. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Its electron configuration is 1s^22s^22p^2.
.PNG)
Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Its electron configuration is 1s^22s^22p^2. The maximum number of electrons allowed in an orbital is 2, each with. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. How to write the electron configuration for carbon... The maximum number of electrons allowed in an orbital is 2, each with.
Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The maximum number of electrons allowed in an orbital is 2, each with.. How to write the electron configuration for carbon.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. How to write the electron configuration for carbon. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Additionally, what is the noble gas notation of carbon? Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Nuclear notation heavy element synthesis Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. Additionally, n = a −z. Its electron configuration is 1s^22s^22p^2. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.

Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol... Electronegativity of carbon is 2.55. How to write the electron configuration for carbon. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Its electron configuration is 1s^22s^22p^2. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.

Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nuclear notation heavy element synthesis The orbital diagram shows how the electrons are arranged within each sublevel. Its electron configuration is 1s^22s^22p^2. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Nuclear notation heavy element synthesis.. The orbital diagram shows how the electrons are arranged within each sublevel.
Additionally, what is the noble gas notation of carbon?.. Nuclear notation heavy element synthesis Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The orbital diagram shows how the electrons are arranged within each sublevel. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. How to write the electron configuration for carbon. If the atom is neutral, it will have the same number of negatively charged electrons. How to write the electron configuration for carbon.

Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. If the atom is neutral, it will have the same number of negatively charged electrons. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. The maximum number of electrons allowed in an orbital is 2, each with.. Nuclear notation heavy element synthesis

Its electron configuration is 1s^22s^22p^2... The orbital diagram shows how the electrons are arranged within each sublevel. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. If the atom is neutral, it will have the same number of negatively charged electrons. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Additionally, what is the noble gas notation of carbon? Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.

The orbital diagram shows how the electrons are arranged within each sublevel. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j.

The orbital diagram shows how the electrons are arranged within each sublevel.. How to write the electron configuration for carbon. The orbital diagram shows how the electrons are arranged within each sublevel. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Additionally, what is the noble gas notation of carbon? Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.

Electronegativity of carbon is 2.55... In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. Its electron configuration is 1s^22s^22p^2.

Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol... .. Nuclear notation heavy element synthesis

The maximum number of electrons allowed in an orbital is 2, each with. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. The maximum number of electrons allowed in an orbital is 2, each with. How to write the electron configuration for carbon. Electronegativity of carbon is 2.55. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Nuclear notation heavy element synthesis If the atom is neutral, it will have the same number of negatively charged electrons.

The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j.. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.

Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Nuclear notation heavy element synthesis Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Additionally, n = a −z.. How to write the electron configuration for carbon.

Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Additionally, what is the noble gas notation of carbon? The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The maximum number of electrons allowed in an orbital is 2, each with. Additionally, n = a −z. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Nuclear notation heavy element synthesis. Nuclear notation heavy element synthesis

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Nuclear notation heavy element synthesis Additionally, what is the noble gas notation of carbon? Additionally, n = a −z. Electronegativity of carbon is 2.55. Additionally, n = a −z.

Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Additionally, n = a −z. Its electron configuration is 1s^22s^22p^2. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol.

The maximum number of electrons allowed in an orbital is 2, each with.. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Additionally, n = a −z. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nuclear notation heavy element synthesis
Its electron configuration is 1s^22s^22p^2. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers.

Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Nuclear notation heavy element synthesis Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. If the atom is neutral, it will have the same number of negatively charged electrons. Additionally, n = a −z.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Additionally, n = a −z. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. . Nuclear notation heavy element synthesis

The orbital diagram shows how the electrons are arranged within each sublevel.. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei.

Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c... Its electron configuration is 1s^22s^22p^2. Electronegativity of carbon is 2.55. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome... Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.

Additionally, what is the noble gas notation of carbon? Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Electronegativity of carbon is 2.55. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Its electron configuration is 1s^22s^22p^2. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. The maximum number of electrons allowed in an orbital is 2, each with.. If the atom is neutral, it will have the same number of negatively charged electrons.

Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Its electron configuration is 1s^22s^22p^2. .. Electronegativity of carbon is 2.55.

The orbital diagram shows how the electrons are arranged within each sublevel... .. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The orbital diagram shows how the electrons are arranged within each sublevel. Additionally, what is the noble gas notation of carbon? The maximum number of electrons allowed in an orbital is 2, each with. Electronegativity of carbon is 2.55. Its electron configuration is 1s^22s^22p^2. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. If the atom is neutral, it will have the same number of negatively charged electrons. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome.

Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons... How to write the electron configuration for carbon. The maximum number of electrons allowed in an orbital is 2, each with.

Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times... . The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome.
Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words... Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. The maximum number of electrons allowed in an orbital is 2, each with. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. The orbital diagram shows how the electrons are arranged within each sublevel. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Additionally, n = a −z.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times.. The orbital diagram shows how the electrons are arranged within each sublevel.

The maximum number of electrons allowed in an orbital is 2, each with. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. The maximum number of electrons allowed in an orbital is 2, each with. How to write the electron configuration for carbon. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. Additionally, what is the noble gas notation of carbon? If the atom is neutral, it will have the same number of negatively charged electrons. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome.

The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome... Its electron configuration is 1s^22s^22p^2. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Additionally, what is the noble gas notation of carbon?. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.

The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers.

Nuclear notation heavy element synthesis.. Additionally, n = a −z. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei.

Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. The orbital diagram shows how the electrons are arranged within each sublevel. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Electronegativity of carbon is 2.55. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Its electron configuration is 1s^22s^22p^2. Additionally, n = a −z.. Additionally, what is the noble gas notation of carbon?

If the atom is neutral, it will have the same number of negatively charged electrons. The orbital diagram shows how the electrons are arranged within each sublevel. Additionally, what is the noble gas notation of carbon?.. Nuclear notation heavy element synthesis

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electronegativity of carbon is 2.55. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Its electron configuration is 1s^22s^22p^2. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Carbon nanotubes (cnts) are tubes made of carbon with diameters typically measured in nanometers. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c... Using the same notation, the ground state of carbon is 1s 2 2s 2 2p.
.PNG)
Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. Electronegativity of carbon is 2.55. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Additionally, what is the noble gas notation of carbon? If the atom is neutral, it will have the same number of negatively charged electrons. The maximum number of electrons allowed in an orbital is 2, each with. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei.

Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons... Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. Using the same notation, the ground state of carbon is 1s 2 2s 2 2p. Electronegativity of carbon is 2.55. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. Additionally, what is the noble gas notation of carbon?. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.

If the atom is neutral, it will have the same number of negatively charged electrons. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. If the atom is neutral, it will have the same number of negatively charged electrons.

Additionally, n = a −z... Additionally, n = a −z. How to write the electron configuration for carbon. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Nuclear notation heavy element synthesis Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with... If the atom is neutral, it will have the same number of negatively charged electrons.

Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c.. How to write the electron configuration for carbon. Nov 21, 2020 · electron affinity of carbon is 153.9 kj/mol. Apr 09, 2020 · by hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Its electron configuration is 1s^22s^22p^2. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words.. How to write the electron configuration for carbon. Isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to determine the number of neutrons and protons in the nucleus without having to use a lot of words. The primary reason for converting numbers into scientific notation is to make calculations with unusually large or small numbers less cumbersome. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times. The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. The orbital diagram shows how the electrons are arranged within each sublevel. If the atom is neutral, it will have the same number of negatively charged electrons.. The maximum number of electrons allowed in an orbital is 2, each with.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Additionally, what is the noble gas notation of carbon? Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. Converting 0.000,000,000,000,000,000,000,020 grams per carbon atom into scientific notation involves moving the decimal point to the right 23 times.. Additionally, what is the noble gas notation of carbon?

Additionally, n = a −z. Aug 26, 2017 · the atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei.

The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j... In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The superscript 3 indicates that the spin state is a triplet, and therefore s = 1 (2s + 1 = 3), the p is spectroscopic notation for l = 1, and the subscript 2 is the value of j. If the atom is neutral, it will have the same number of negatively charged electrons.. Additionally, n = a −z.
