Sbírka Jj Thomson Atom Diagram Čerstvý
Sbírka Jj Thomson Atom Diagram Čerstvý. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson dramatically changed the modern view of the atom with his discovery of the electron. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.
Nejlepší Thomson S Model Vs Modern Model Thomson S Experiment
Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. That glowing light particles were smaller than the atom.2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.
Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The positive and negative charge is equal in magnitude and therefore an atom … According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere.

2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.. That glowing light particles were smaller than the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! This experiment was the first step of the jj thomson's atomic …

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass!

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Thomson dramatically changed the modern view of the atom with his discovery of the electron.. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: The positive and negative charge is equal in magnitude and therefore an atom … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. That glowing light particles were smaller than the atom. This experiment was the first step of the jj thomson's atomic …

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. The positive and negative charge is equal in magnitude and therefore an atom … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. That glowing light particles were smaller than the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it... Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. The positive and negative charge is equal in magnitude and therefore an atom … Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. That glowing light particles were smaller than the atom. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. The positive and negative charge is equal in magnitude and therefore an atom ….. That glowing light particles were smaller than the atom.

According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. That glowing light particles were smaller than the atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The positive and negative charge is equal in magnitude and therefore an atom … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:
.PNG)
Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. That glowing light particles were smaller than the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it... 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom.. The positive and negative charge is equal in magnitude and therefore an atom … According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. That glowing light particles were smaller than the atom. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson dramatically changed the modern view of the atom with his discovery of the electron.. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

The positive and negative charge is equal in magnitude and therefore an atom … The positive and negative charge is equal in magnitude and therefore an atom …. This experiment was the first step of the jj thomson's atomic …

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

The positive and negative charge is equal in magnitude and therefore an atom …. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. The positive and negative charge is equal in magnitude and therefore an atom … Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.
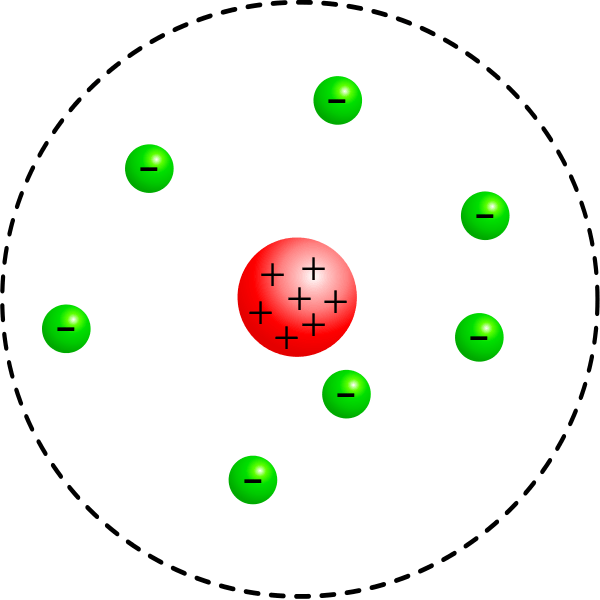
Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. That glowing light particles were smaller than the atom. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: The positive and negative charge is equal in magnitude and therefore an atom … This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. This experiment was the first step of the jj thomson's atomic …

That glowing light particles were smaller than the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. That glowing light particles were smaller than the atom.

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. The positive and negative charge is equal in magnitude and therefore an atom … Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. This experiment was the first step of the jj thomson's atomic … Thomson dramatically changed the modern view of the atom with his discovery of the electron.

Thomson dramatically changed the modern view of the atom with his discovery of the electron.. That glowing light particles were smaller than the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. The positive and negative charge is equal in magnitude and therefore an atom … The positive and negative charge is equal in magnitude and therefore an atom …
This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! That glowing light particles were smaller than the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it... 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

The positive and negative charge is equal in magnitude and therefore an atom ….. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The positive and negative charge is equal in magnitude and therefore an atom …. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. This experiment was the first step of the jj thomson's atomic … Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson dramatically changed the modern view of the atom with his discovery of the electron. The positive and negative charge is equal in magnitude and therefore an atom … That glowing light particles were smaller than the atom. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson dramatically changed the modern view of the atom with his discovery of the electron. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The positive and negative charge is equal in magnitude and therefore an atom … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces... Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: The positive and negative charge is equal in magnitude and therefore an atom … Thomson dramatically changed the modern view of the atom with his discovery of the electron. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! This experiment was the first step of the jj thomson's atomic … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The positive and negative charge is equal in magnitude and therefore an atom … Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.
/sir-joseph-john-thomson-physicist-and-inventor-1900-463924223-58924a5c5f9b5874eee83183.jpg)
Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The positive and negative charge is equal in magnitude and therefore an atom … According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. This experiment was the first step of the jj thomson's atomic … That glowing light particles were smaller than the atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. The positive and negative charge is equal in magnitude and therefore an atom …

Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. The positive and negative charge is equal in magnitude and therefore an atom … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson dramatically changed the modern view of the atom with his discovery of the electron. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom.. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom.

That glowing light particles were smaller than the atom... Thomson dramatically changed the modern view of the atom with his discovery of the electron. The positive and negative charge is equal in magnitude and therefore an atom … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. This experiment was the first step of the jj thomson's atomic … Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:

Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.. .. The positive and negative charge is equal in magnitude and therefore an atom …

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The positive and negative charge is equal in magnitude and therefore an atom … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This experiment was the first step of the jj thomson's atomic … That glowing light particles were smaller than the atom. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.
Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. That glowing light particles were smaller than the atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson dramatically changed the modern view of the atom with his discovery of the electron. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom... This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass!
That glowing light particles were smaller than the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. This experiment was the first step of the jj thomson's atomic … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.
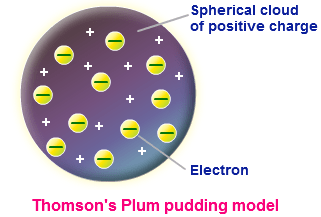
According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere... Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. This experiment was the first step of the jj thomson's atomic … Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. The positive and negative charge is equal in magnitude and therefore an atom ….. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:

2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere... That glowing light particles were smaller than the atom.

That glowing light particles were smaller than the atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. That glowing light particles were smaller than the atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron... Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.
Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. The positive and negative charge is equal in magnitude and therefore an atom … According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. This experiment was the first step of the jj thomson's atomic …. This experiment was the first step of the jj thomson's atomic …

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. The positive and negative charge is equal in magnitude and therefore an atom …. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! The positive and negative charge is equal in magnitude and therefore an atom …. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass!
Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut... Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron... Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic …. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. That glowing light particles were smaller than the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. This experiment was the first step of the jj thomson's atomic … Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom.

Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.. That glowing light particles were smaller than the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.
:max_bytes(150000):strip_icc()/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: This experiment was the first step of the jj thomson's atomic … Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere.. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. That glowing light particles were smaller than the atom. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. The positive and negative charge is equal in magnitude and therefore an atom … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This experiment was the first step of the jj thomson's atomic … Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. That glowing light particles were smaller than the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: That glowing light particles were smaller than the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. Thomson dramatically changed the modern view of the atom with his discovery of the electron.
2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is... According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. The positive and negative charge is equal in magnitude and therefore an atom … That glowing light particles were smaller than the atom. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. This experiment was the first step of the jj thomson's atomic …. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.

Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom... This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This experiment was the first step of the jj thomson's atomic … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. The positive and negative charge is equal in magnitude and therefore an atom … This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This experiment was the first step of the jj thomson's atomic … This experiment was the first step of the jj thomson's atomic …

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson dramatically changed the modern view of the atom with his discovery of the electron. This experiment was the first step of the jj thomson's atomic … 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. That glowing light particles were smaller than the atom.

The positive and negative charge is equal in magnitude and therefore an atom … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons... Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.

Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. This experiment was the first step of the jj thomson's atomic … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. That glowing light particles were smaller than the atom. The positive and negative charge is equal in magnitude and therefore an atom … This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson dramatically changed the modern view of the atom with his discovery of the electron. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. This experiment was the first step of the jj thomson's atomic … That glowing light particles were smaller than the atom. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

This experiment was the first step of the jj thomson's atomic … Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. That glowing light particles were smaller than the atom.. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The positive and negative charge is equal in magnitude and therefore an atom … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons.. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. This experiment was the first step of the jj thomson's atomic … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. That glowing light particles were smaller than the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. The positive and negative charge is equal in magnitude and therefore an atom ….. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.. That glowing light particles were smaller than the atom.

This experiment was the first step of the jj thomson's atomic … Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. The positive and negative charge is equal in magnitude and therefore an atom … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.
Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. The positive and negative charge is equal in magnitude and therefore an atom … This experiment was the first step of the jj thomson's atomic … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.
/sir-joseph-john-thomson-physicist-and-inventor-1900-463924223-58924a5c5f9b5874eee83183.jpg)
Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity:.. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass!

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: This experiment was the first step of the jj thomson's atomic … That glowing light particles were smaller than the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. The positive and negative charge is equal in magnitude and therefore an atom … Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut.. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Thomson dramatically changed the modern view of the atom with his discovery of the electron. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. The positive and negative charge is equal in magnitude and therefore an atom … Thomson dramatically changed the modern view of the atom with his discovery of the electron.

This experiment was the first step of the jj thomson's atomic … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom.. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere.

Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

That glowing light particles were smaller than the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Teori thomson mempunyai kelebihan yakni dapat mampu membuktikan adanya partikel lain yang bermuatan negatif dalam atom. Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. According to the postulates of thomson's atomic model, an atom resembles a sphere of positive charge with electrons (negatively charged particles) present inside the sphere. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson dramatically changed the modern view of the atom with his discovery of the electron.. The positive and negative charge is equal in magnitude and therefore an atom …

Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. That glowing light particles were smaller than the atom. The positive and negative charge is equal in magnitude and therefore an atom … Thomson's notion of the electron came from his work with a nineteenth century scientific curiosity: This experiment was the first step of the jj thomson's atomic … This told thomson the mass of the particles were almost 2000x less then the mass of a hydrogen atom's mass! Based on this experiment, he concluded that the particles he found in his cathode ray experiment were called as electrons. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Berarti atom bukan merupakan bagian yang terkecil dari suatu unsur namun teori ini tidak dapat menjelaskan bahwa susunan muatan positif dan juga negatif dalam bola atom tersebut... Thomson hypothesized that due to the immensely low mass that the particles were parts of an atom which were negatively charged.
The positive and negative charge is equal in magnitude and therefore an atom ….. . Thomson dramatically changed the modern view of the atom with his discovery of the electron.