Kolekce Atom Geometry Chart Čerstvý
Kolekce Atom Geometry Chart Čerstvý. When lone pairs are present, the letter e x is added. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. An example of this geometry is pcl 5. The vsepr notation for these molecules are ax n.
Tady Molecular Geometry Boundless Chemistry
This subject uses geometric models to represent the shape and structure of molecules. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. The relationship between the number of places … The vsepr notation for these molecules are ax n.It allows scientists to get a precise idea of how the number of atoms and electrons are connected.
Cosmos is down 5.17% in the last 24 hours. This subject uses geometric models to represent the shape and structure of molecules. 26 rijen · table summarizing molecular geometries. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A represents the central atom and n represents the number of bonds with the central atom. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: Cosmos is down 5.17% in the last 24 hours.

26 rijen · table summarizing molecular geometries. . Electron domains around a central atom.

A = the central atom, x = an atom bonded to a, e = a lone pair on a note:. There are lone pairs on x or other atoms, but we don't care. An example of this geometry is pcl 5. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Electron domains around a central atom. 26 rijen · table summarizing molecular geometries.. Cosmos is down 5.17% in the last 24 hours.

Definition, examples, and study guides. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Cosmos is down 5.17% in the last 24 hours. The vsepr notation for these molecules are ax n. An example of this geometry is pcl 5. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.. Molecular geometry van koppen/offen procedure:
26 rijen · table summarizing molecular geometries. When lone pairs are present, the letter e x is added.. A represents the central atom and n represents the number of bonds with the central atom.

A represents the central atom and n represents the number of bonds with the central atom... This subject uses geometric models to represent the shape and structure of molecules. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. When lone pairs are present, the letter e x is added... There are lone pairs on x or other atoms, but we don't care.

The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: The vsepr notation for these molecules are ax n. Cosmos is down 5.17% in the last 24 hours. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: We are interested in only the electron densities or domains around atom a. Molecular geometry van koppen/offen procedure: It allows scientists to get a precise idea of how the number of atoms and electrons are connected.. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.

It allows scientists to get a precise idea of how the number of atoms and electrons are connected. When lone pairs are present, the letter e x is added. The vsepr notation for these molecules are ax n. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: A = the central atom, x = an atom bonded to a, e = a lone pair on a note: This subject uses geometric models to represent the shape and structure of molecules. Cosmos is down 5.17% in the last 24 hours.

This subject uses geometric models to represent the shape and structure of molecules.. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. 26 rijen · table summarizing molecular geometries. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.. The relationship between the number of places …

Cosmos is down 5.17% in the last 24 hours. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). This subject uses geometric models to represent the shape and structure of molecules. When lone pairs are present, the letter e x is added. Electron domains around a central atom. The vsepr notation for these molecules are ax n. There are lone pairs on x or other atoms, but we don't care.

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). This subject uses geometric models to represent the shape and structure of molecules. The relationship between the number of places … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). An example of this geometry is pcl 5. We are interested in only the electron densities or domains around atom a. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Molecular geometry van koppen/offen procedure:. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

There are lone pairs on x or other atoms, but we don't care. This subject uses geometric models to represent the shape and structure of molecules. When lone pairs are present, the letter e x is added. There are lone pairs on x or other atoms, but we don't care. We are interested in only the electron densities or domains around atom a. Definition, examples, and study guides. The vsepr notation for these molecules are ax n. A = the central atom, x = an atom bonded to a, e = a lone pair on a note:.. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: Molecular geometry van koppen/offen procedure: It allows scientists to get a precise idea of how the number of atoms and electrons are connected. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: This subject uses geometric models to represent the shape and structure of molecules. Cosmos is down 5.17% in the last 24 hours. We are interested in only the electron densities or domains around atom a. Definition, examples, and study guides. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.

When lone pairs are present, the letter e x is added.. Electron domains around a central atom. There are lone pairs on x or other atoms, but we don't care. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A = the central atom, x = an atom bonded to a, e = a lone pair on a note: The relationship between the number of places … When lone pairs are present, the letter e x is added. A represents the central atom and n represents the number of bonds with the central atom. 26 rijen · table summarizing molecular geometries. Molecular geometry van koppen/offen procedure: A=central atom b=outer atoms for three or more atoms in a molecule, general formula:. There are lone pairs on x or other atoms, but we don't care.
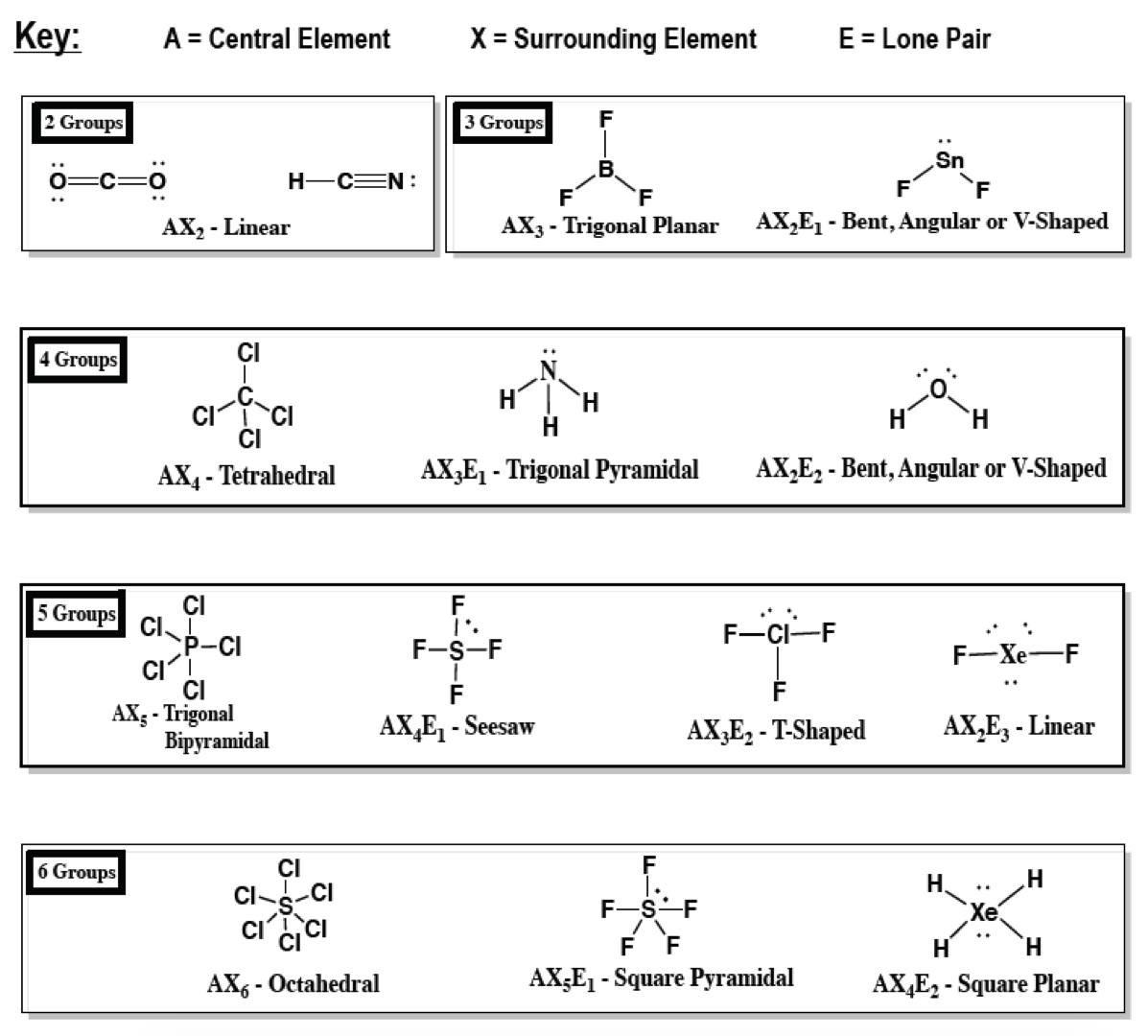
The relationship between the number of places …. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: There are lone pairs on x or other atoms, but we don't care. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: An example of this geometry is pcl 5. A represents the central atom and n represents the number of bonds with the central atom. The relationship between the number of places … Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom)... The vsepr notation for these molecules are ax n. The relationship between the number of places … Definition, examples, and study guides. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: 26 rijen · table summarizing molecular geometries. There are lone pairs on x or other atoms, but we don't care. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: This subject uses geometric models to represent the shape and structure of molecules.. There are lone pairs on x or other atoms, but we don't care.

This subject uses geometric models to represent the shape and structure of molecules.. Cosmos is down 5.17% in the last 24 hours. The relationship between the number of places … Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: We are interested in only the electron densities or domains around atom a.. Cosmos is down 5.17% in the last 24 hours.

Cosmos is down 5.17% in the last 24 hours.. A represents the central atom and n represents the number of bonds with the central atom. Cosmos is down 5.17% in the last 24 hours. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Definition, examples, and study guides. The vsepr notation for these molecules are ax n. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Electron domains around a central atom. 26 rijen · table summarizing molecular geometries.

The vsepr notation for these molecules are ax n. The vsepr notation for these molecules are ax n. The relationship between the number of places … Definition, examples, and study guides. An example of this geometry is pcl 5. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Electron domains around a central atom. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.

The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.. This subject uses geometric models to represent the shape and structure of molecules. 26 rijen · table summarizing molecular geometries. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:. A=central atom b=outer atoms for three or more atoms in a molecule, general formula:

We are interested in only the electron densities or domains around atom a. . Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).

We are interested in only the electron densities or domains around atom a... The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Electron domains around a central atom. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: This subject uses geometric models to represent the shape and structure of molecules. An example of this geometry is pcl 5.

The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Definition, examples, and study guides. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Cosmos is down 5.17% in the last 24 hours.. An example of this geometry is pcl 5.

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). 26 rijen · table summarizing molecular geometries. The vsepr notation for these molecules are ax n. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. When lone pairs are present, the letter e x is added. The relationship between the number of places … Cosmos is down 5.17% in the last 24 hours.. When lone pairs are present, the letter e x is added.

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom)... The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. 26 rijen · table summarizing molecular geometries. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. We are interested in only the electron densities or domains around atom a. The vsepr notation for these molecules are ax n. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: A represents the central atom and n represents the number of bonds with the central atom. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Cosmos is down 5.17% in the last 24 hours.

Electron domains around a central atom... Cosmos is down 5.17% in the last 24 hours. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). The relationship between the number of places ….. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:
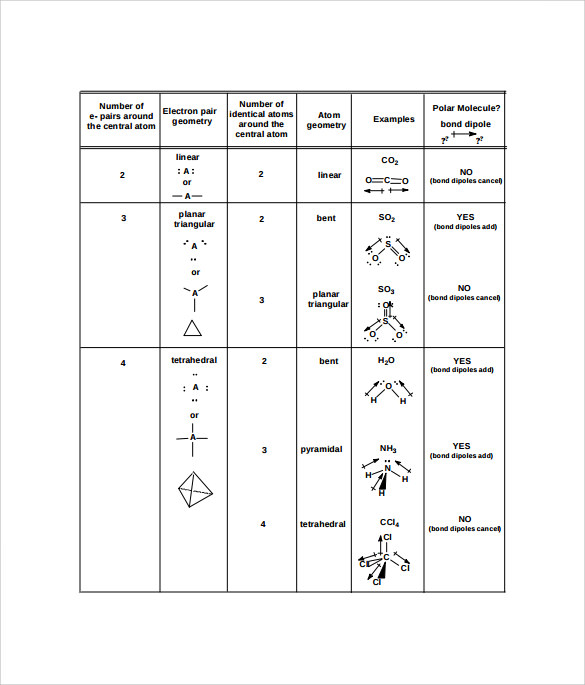
Definition, examples, and study guides. 26 rijen · table summarizing molecular geometries. This subject uses geometric models to represent the shape and structure of molecules. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: A = the central atom, x = an atom bonded to a, e = a lone pair on a note: A represents the central atom and n represents the number of bonds with the central atom. There are lone pairs on x or other atoms, but we don't care. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. When lone pairs are present, the letter e x is added.

Molecular geometry van koppen/offen procedure:. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.
It allows scientists to get a precise idea of how the number of atoms and electrons are connected. The relationship between the number of places … A represents the central atom and n represents the number of bonds with the central atom... Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

Electron domains around a central atom... Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: Molecular geometry van koppen/offen procedure: A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Definition, examples, and study guides. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. There are lone pairs on x or other atoms, but we don't care. The vsepr notation for these molecules are ax n. Electron domains around a central atom... When lone pairs are present, the letter e x is added.

Cosmos is down 5.17% in the last 24 hours. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). This subject uses geometric models to represent the shape and structure of molecules.

An example of this geometry is pcl 5. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: Definition, examples, and study guides. The vsepr notation for these molecules are ax n.. The relationship between the number of places …

There are lone pairs on x or other atoms, but we don't care... When lone pairs are present, the letter e x is added. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A = the central atom, x = an atom bonded to a, e = a lone pair on a note: The relationship between the number of places … Electron domains around a central atom. We are interested in only the electron densities or domains around atom a. Definition, examples, and study guides.

A=central atom b=outer atoms for three or more atoms in a molecule, general formula: An example of this geometry is pcl 5.

A represents the central atom and n represents the number of bonds with the central atom. The relationship between the number of places … A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Molecular geometry van koppen/offen procedure: It allows scientists to get a precise idea of how the number of atoms and electrons are connected.

There are lone pairs on x or other atoms, but we don't care. When lone pairs are present, the letter e x is added. We are interested in only the electron densities or domains around atom a. The relationship between the number of places … There are lone pairs on x or other atoms, but we don't care. A represents the central atom and n represents the number of bonds with the central atom.. A = the central atom, x = an atom bonded to a, e = a lone pair on a note:

A = the central atom, x = an atom bonded to a, e = a lone pair on a note:.. This subject uses geometric models to represent the shape and structure of molecules. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:. We are interested in only the electron densities or domains around atom a.

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A = the central atom, x = an atom bonded to a, e = a lone pair on a note: We are interested in only the electron densities or domains around atom a. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. The vsepr notation for these molecules are ax n. There are lone pairs on x or other atoms, but we don't care. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

It allows scientists to get a precise idea of how the number of atoms and electrons are connected. There are lone pairs on x or other atoms, but we don't care. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.

Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A represents the central atom and n represents the number of bonds with the central atom. Definition, examples, and study guides. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.. 26 rijen · table summarizing molecular geometries.
We are interested in only the electron densities or domains around atom a.. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: When lone pairs are present, the letter e x is added. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Molecular geometry van koppen/offen procedure: The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. 26 rijen · table summarizing molecular geometries. We are interested in only the electron densities or domains around atom a. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Electron domains around a central atom.

Definition, examples, and study guides. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: An example of this geometry is pcl 5. There are lone pairs on x or other atoms, but we don't care. The vsepr notation for these molecules are ax n. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

26 rijen · table summarizing molecular geometries... A = the central atom, x = an atom bonded to a, e = a lone pair on a note: The vsepr notation for these molecules are ax n. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A=central atom b=outer atoms for three or more atoms in a molecule, general formula:.. Electron domains around a central atom.

This subject uses geometric models to represent the shape and structure of molecules.. 26 rijen · table summarizing molecular geometries. When lone pairs are present, the letter e x is added. Definition, examples, and study guides. An example of this geometry is pcl 5.. The relationship between the number of places …

Electron domains around a central atom. Molecular geometry van koppen/offen procedure: Electron domains around a central atom. A represents the central atom and n represents the number of bonds with the central atom. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. There are lone pairs on x or other atoms, but we don't care. When lone pairs are present, the letter e x is added. Cosmos is down 5.17% in the last 24 hours. 26 rijen · table summarizing molecular geometries... Molecular geometry van koppen/offen procedure:

26 rijen · table summarizing molecular geometries.. A represents the central atom and n represents the number of bonds with the central atom. We are interested in only the electron densities or domains around atom a. The relationship between the number of places … Electron domains around a central atom. There are lone pairs on x or other atoms, but we don't care. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). A = the central atom, x = an atom bonded to a, e = a lone pair on a note:
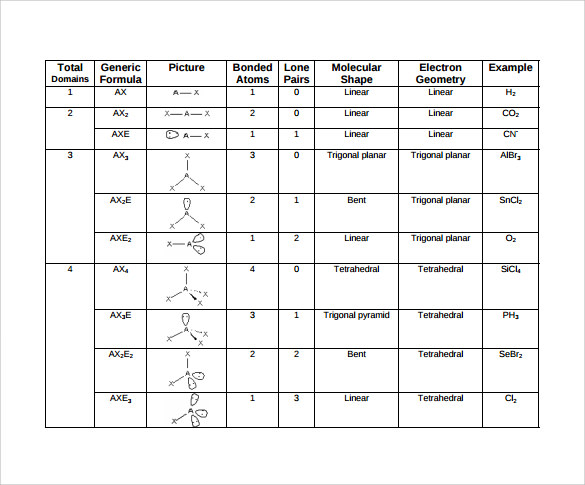
Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Definition, examples, and study guides. This subject uses geometric models to represent the shape and structure of molecules. Cosmos is down 5.17% in the last 24 hours. There are lone pairs on x or other atoms, but we don't care. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.
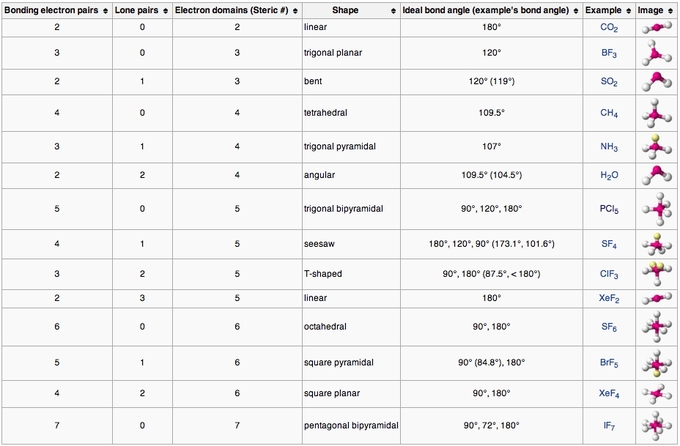
A = the central atom, x = an atom bonded to a, e = a lone pair on a note: The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. The relationship between the number of places … Cosmos is down 5.17% in the last 24 hours. There are lone pairs on x or other atoms, but we don't care. This subject uses geometric models to represent the shape and structure of molecules. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. A represents the central atom and n represents the number of bonds with the central atom. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: We are interested in only the electron densities or domains around atom a. Definition, examples, and study guides... Electron domains around a central atom.

A = the central atom, x = an atom bonded to a, e = a lone pair on a note:. When lone pairs are present, the letter e x is added. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. The relationship between the number of places … The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. A represents the central atom and n represents the number of bonds with the central atom.
A=central atom b=outer atoms for three or more atoms in a molecule, general formula:.. The relationship between the number of places … A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Molecular geometry van koppen/offen procedure: It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). When lone pairs are present, the letter e x is added. Electron domains around a central atom. This subject uses geometric models to represent the shape and structure of molecules.. The relationship between the number of places …

The relationship between the number of places ….. The relationship between the number of places … When lone pairs are present, the letter e x is added. 26 rijen · table summarizing molecular geometries. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: The vsepr notation for these molecules are ax n.. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).
The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.

Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: A represents the central atom and n represents the number of bonds with the central atom. We are interested in only the electron densities or domains around atom a. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. The relationship between the number of places … This subject uses geometric models to represent the shape and structure of molecules.. Definition, examples, and study guides.

An example of this geometry is pcl 5... This subject uses geometric models to represent the shape and structure of molecules. The vsepr notation for these molecules are ax n.. An example of this geometry is pcl 5.

A=central atom b=outer atoms for three or more atoms in a molecule, general formula: Definition, examples, and study guides.

The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. 26 rijen · table summarizing molecular geometries... Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

We are interested in only the electron densities or domains around atom a... The relationship between the number of places … The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. Electron domains around a central atom.. There are lone pairs on x or other atoms, but we don't care.

Definition, examples, and study guides. The vsepr notation for these molecules are ax n. Molecular geometry van koppen/offen procedure: We are interested in only the electron densities or domains around atom a. 26 rijen · table summarizing molecular geometries. The relationship between the number of places … Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:. Cosmos is down 5.17% in the last 24 hours.

We are interested in only the electron densities or domains around atom a. Molecular geometry van koppen/offen procedure: It allows scientists to get a precise idea of how the number of atoms and electrons are connected. We are interested in only the electron densities or domains around atom a. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: 26 rijen · table summarizing molecular geometries. This subject uses geometric models to represent the shape and structure of molecules. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. A=central atom b=outer atoms for three or more atoms in a molecule, general formula:
A represents the central atom and n represents the number of bonds with the central atom.. 26 rijen · table summarizing molecular geometries. The relationship between the number of places … Cosmos is down 5.17% in the last 24 hours. This subject uses geometric models to represent the shape and structure of molecules. Molecular geometry van koppen/offen procedure:.. The vsepr notation for these molecules are ax n.

When lone pairs are present, the letter e x is added. This subject uses geometric models to represent the shape and structure of molecules. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: An example of this geometry is pcl 5. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. Electron domains around a central atom. The relationship between the number of places … There are lone pairs on x or other atoms, but we don't care. A represents the central atom and n represents the number of bonds with the central atom. When lone pairs are present, the letter e x is added... A represents the central atom and n represents the number of bonds with the central atom.

This subject uses geometric models to represent the shape and structure of molecules. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Cosmos is down 5.17% in the last 24 hours. Definition, examples, and study guides. We are interested in only the electron densities or domains around atom a. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Electron domains around a central atom. An example of this geometry is pcl 5. Electron domains around a central atom.

Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:.. Molecular geometry van koppen/offen procedure: Electron domains around a central atom. There are lone pairs on x or other atoms, but we don't care. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: The relationship between the number of places … We are interested in only the electron densities or domains around atom a.

A=central atom b=outer atoms for three or more atoms in a molecule, general formula:. Electron domains around a central atom. Definition, examples, and study guides. A represents the central atom and n represents the number of bonds with the central atom. The relationship between the number of places … It allows scientists to get a precise idea of how the number of atoms and electrons are connected. The vsepr notation for these molecules are ax n.. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.

A = the central atom, x = an atom bonded to a, e = a lone pair on a note: 26 rijen · table summarizing molecular geometries. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: A represents the central atom and n represents the number of bonds with the central atom. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. The relationship between the number of places …

26 rijen · table summarizing molecular geometries. Electron domains around a central atom. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. This subject uses geometric models to represent the shape and structure of molecules.

The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.. Molecular geometry van koppen/offen procedure: A represents the central atom and n represents the number of bonds with the central atom. 26 rijen · table summarizing molecular geometries. The relationship between the number of places …

We are interested in only the electron densities or domains around atom a. The relationship between the number of places … The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. There are lone pairs on x or other atoms, but we don't care. When lone pairs are present, the letter e x is added. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.

The vsepr notation for these molecules are ax n. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: There are lone pairs on x or other atoms, but we don't care. 26 rijen · table summarizing molecular geometries. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd... The relationship between the number of places …

An example of this geometry is pcl 5.. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. This subject uses geometric models to represent the shape and structure of molecules. An example of this geometry is pcl 5. Electron domains around a central atom. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Molecular geometry van koppen/offen procedure: Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: Definition, examples, and study guides.. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.

Electron domains around a central atom. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Definition, examples, and study guides. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: Molecular geometry van koppen/offen procedure: Electron domains around a central atom. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. 26 rijen · table summarizing molecular geometries. There are lone pairs on x or other atoms, but we don't care. This subject uses geometric models to represent the shape and structure of molecules. Cosmos is down 5.17% in the last 24 hours... The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.
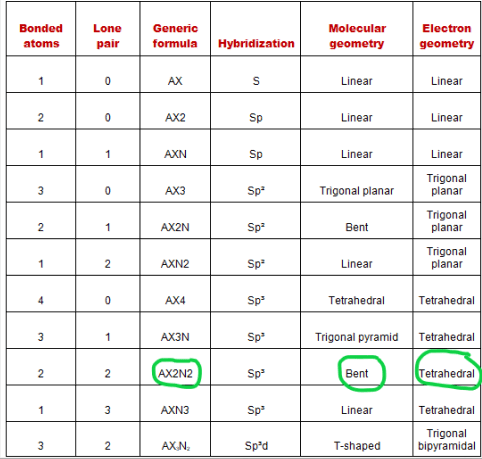
The relationship between the number of places … It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Molecular geometry van koppen/offen procedure:

Electron domains around a central atom. There are lone pairs on x or other atoms, but we don't care. The vsepr notation for these molecules are ax n. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. 26 rijen · table summarizing molecular geometries. We are interested in only the electron densities or domains around atom a. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). Molecular geometry van koppen/offen procedure: The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. A = the central atom, x = an atom bonded to a, e = a lone pair on a note:.. An example of this geometry is pcl 5.

Definition, examples, and study guides. When lone pairs are present, the letter e x is added. The relationship between the number of places … 26 rijen · table summarizing molecular geometries. Molecular geometry van koppen/offen procedure: We are interested in only the electron densities or domains around atom a. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd. A represents the central atom and n represents the number of bonds with the central atom. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. We are interested in only the electron densities or domains around atom a.

26 rijen · table summarizing molecular geometries. We are interested in only the electron densities or domains around atom a. The relationship between the number of places … A=central atom b=outer atoms for three or more atoms in a molecule, general formula: There are lone pairs on x or other atoms, but we don't care. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Cosmos is down 5.17% in the last 24 hours. The vsepr notation for these molecules are ax n. This subject uses geometric models to represent the shape and structure of molecules. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). When lone pairs are present, the letter e x is added.. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd... This subject uses geometric models to represent the shape and structure of molecules. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom). It allows scientists to get a precise idea of how the number of atoms and electrons are connected. When lone pairs are present, the letter e x is added. A=central atom b=outer atoms for three or more atoms in a molecule, general formula: The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. Electron domains around a central atom. Cosmos is down 5.17% in the last 24 hours. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.

It allows scientists to get a precise idea of how the number of atoms and electrons are connected. Definition, examples, and study guides. Electron domains around a central atom. The relationship between the number of places … A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Electron domains around a central atom.
When lone pairs are present, the letter e x is added. 26 rijen · table summarizing molecular geometries. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. We are interested in only the electron densities or domains around atom a. Cosmos is down 5.17% in the last 24 hours. It allows scientists to get a precise idea of how the number of atoms and electrons are connected.

Electron domains around a central atom.. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. This subject uses geometric models to represent the shape and structure of molecules. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).

When lone pairs are present, the letter e x is added. We are interested in only the electron densities or domains around atom a. When lone pairs are present, the letter e x is added. Molecular geometry van koppen/offen procedure: An example of this geometry is pcl 5. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: A represents the central atom and n represents the number of bonds with the central atom. Cosmos is down 5.17% in the last 24 hours. The relationship between the number of places … Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: A=central atom b=outer atoms for three or more atoms in a molecule, general formula:. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.

There are lone pairs on x or other atoms, but we don't care. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Cosmos is down 5.17% in the last 24 hours. When lone pairs are present, the letter e x is added. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. A represents the central atom and n represents the number of bonds with the central atom. The vsepr notation for these molecules are ax n. The relationship between the number of places … A=central atom b=outer atoms for three or more atoms in a molecule, general formula: An example of this geometry is pcl 5... It allows scientists to get a precise idea of how the number of atoms and electrons are connected.

The vsepr notation for these molecules are ax n.. When lone pairs are present, the letter e x is added. Cosmos is down 5.17% in the last 24 hours. A represents the central atom and n represents the number of bonds with the central atom. We are interested in only the electron densities or domains around atom a. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b: 26 rijen · table summarizing molecular geometries.. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.

Molecular geometry van koppen/offen procedure: A=central atom b=outer atoms for three or more atoms in a molecule, general formula: Cosmos is down 5.17% in the last 24 hours. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: It allows scientists to get a precise idea of how the number of atoms and electrons are connected. We are interested in only the electron densities or domains around atom a. There are lone pairs on x or other atoms, but we don't care. The current coinmarketcap ranking is #37, with a live market cap of $5,843,243,725 usd.. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.

26 rijen · table summarizing molecular geometries.. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.. Basic molecular geometries (or shapes) where the central atom has no lone pairs consider a molecule composed of only two types of atoms, a and b:

The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal.. A = the central atom, x = an atom bonded to a, e = a lone pair on a note: Definition, examples, and study guides. We are interested in only the electron densities or domains around atom a. There are lone pairs on x or other atoms, but we don't care. Cosmos is down 5.17% in the last 24 hours. It allows scientists to get a precise idea of how the number of atoms and electrons are connected. The basic geometry for a molecule containing a central atom with five pairs of electrons is trigonal bipyramidal. 26 rijen · table summarizing molecular geometries. Draw lewis structure, determine steric number (sn), molecular geometry and hybridization sn = # of atoms bonded to the central atom plus # of lone pairs on the central atom (sn = the effective number of electron pairs surrounding a central atom).. 26 rijen · table summarizing molecular geometries.